The skill and expertise of our scientific team makes JRF Global the perfect choice for all IND-enabling safety studies.
JRF Global’s IND-enabling safety evaluation expertise encompasses a wide range of services in, method development, validation, impurity profiling, impurity isolation, characterization, certification and evaluation, mammalian toxicology, genetic toxicology, drug metabolism, pharmacokinetics, and bio-analysis during the preclinical stage of drug development.
Our teams, led by Ph.D. scientists provide guidance to support your IND submissions. JRF’s scientific teams have hundreds of man-years of experience in conducting the the chemical characterization as well as preclinical studies, focusing on proving the safety and efficacy of the compounds to convince the regulatory bodies to receive the permissions for first in man trials. Many of our valuable customers have successfully submitted their INDs to FDA, EMA and other regulatory bodies.
JRF’s accelerated IND reports:
Timelines are the most critical for the IND submissions and hence, JRF Global has adopted innovative system for accelerated IND evaluation and submission summary reports as well as audited draft reports in order to enable you to plan your submissions in advance.
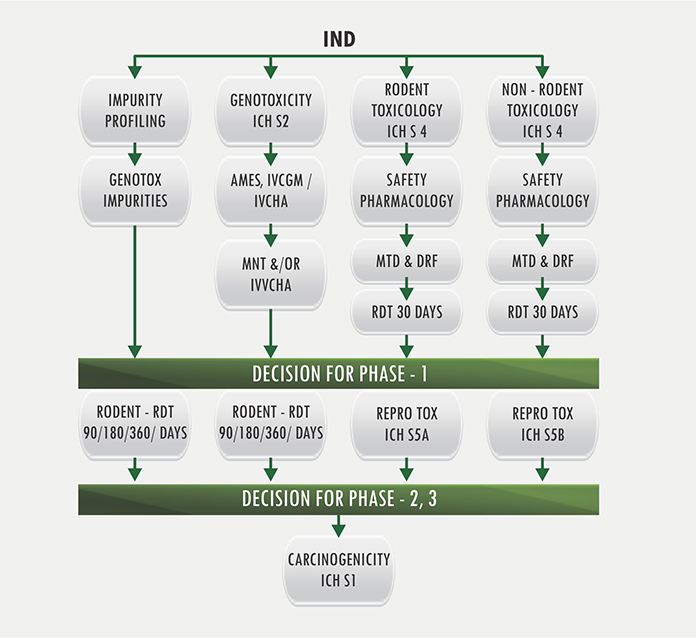
IND enabling Safety Evaluation studies:
-
Mammalian Toxicology
-
Drug Metabolism and Pharmacokinetics
-
Genetic Toxicology
-
Safety Pharmacology
-
Immunotoxicity
-
Neurotoxicity
-
Bio-analysis
Services available at following locations: