TICO (Test Item Control Office)
JRF Global has a state-of-the-art, highly secured and computerised Test Item Control Office (TICO)
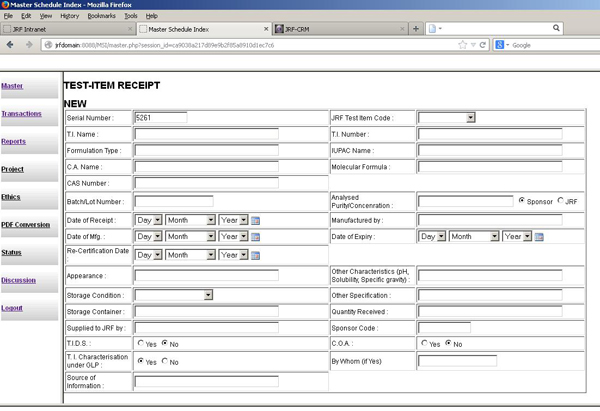
The Test Item Control Office ensures safe receipt of the test item, provides a unique code for each item, sponsors, maintains its mass balance and issue log, and holds the same in environmentally controlled storage till usage, expiry and disposal.
The JRF TICO facility has discrete, well separated areas to receive and store different types of products, such as pharmaceuticals, crop protection products, and other chemicals. The facility is connected with our master schedule software program so the sample receipt is logged into the software along with the sample code.
The TICO ensures that the test item receipt note is sent to the sponsor automatically within 48 hours of safe receipt. Our TICO officers also guide each study sponsor in case of absence of adequate documents to comply with the GLP guidelines requirements.
The TICO facility has well isolated zones for handling Pharmaceuticals, Crop protection products, and other chemicals to ensure elimination of contamination possibilities. They are also responsible for guiding the user/scientists about the hazards involved in handling a given test chemical.
TICO ensures that the test item receipt note is sent to the sponsor within 48 hours after the safe receipt. TICO officers also guide our sponsors in respect of absence of adequate documents to comply with the GLP guidelines.
We have separate Test Item Control Offices for Pharma and non-pharma products. All the test items are stored under suitable conditions based on their stability. The test items are handed over to the study director with guidance in respect of their stability to air and light.